MANAGEMENT CONSULTING. CLINICAL RESEARCH OPTIMIZATION. HEALTH EQUITY THOUGHT LEADERSHIP. INDEPENDENT PERSPECTIVE.
PARTNERSHIP.
New Remedy Consulting provides management and communications consulting expertise to companies in the biotechnology, pharmaceutical, healthcare, and wellness spaces.
With experience at organizations ranging from private start-ups to non-profit organizations to public, global firms, New Remedy provides an independent perspective rooted in 15 years of experience in the life sciences and health industries to our partners.
Extensive experience in clinical research and development, including clinical trial operations, study feasibility, site management and monitoring, and program management.
Thought leadership on the topics of health equity, health disparities, and clinical trial diversity, equity, and inclusion.
Project management and communications expertise with a special focus on clients in the health equity, healthcare, and wellness spaces.
It’s time to ditch the feeling of inauthenticity when working with a consulting partner.
We’ve heard from many people that they’ve felt stranded or frustrated with consultants in the past. Maybe you feel this way too. But it doesn’t have to be. We get it.
-
15 years of hands-on experience in clinical research and development, including clinical trial operations, study feasibility, and site management. Extensive background in management consulting, program oversight, and project management. Key roles in building, leading, and guiding cross-functional and multi-stakeholder teams.
-
Our solutions are built on a diversity of life experience, professional background, personal identity, and thought. We approach challenges with an innovative and direct point of view while flexing to the specific needs and organizational cultures of our clients.
-
Strategy matters. Tactics matter. Words matter. But without a foundation of humility and respect, a collaboration can’t be at its best. This philosophy drives what we do and what makes us authentic partners to our clients.

ABOUT
I’m RACQUEL, a thought leader, a change agent, and a partner.
RACQUEL RACADIO, DrPH, MPH, Founder + Principal Consultant
Racquel has nearly two decades of experience in clinical research, medical affairs, and public health, focused on external partnership development, program oversight, project management, clinical development and operations, and communications. She has worked directly in R&D with and for start-ups, large clinical research organizations, global biotechnology/pharmaceutical companies, and non-profit consortia as a medical affairs leader, a clinical development professional, and as an independent consultant.
Throughout her career, Racquel’s focus has always centered on improving health outcomes and access for communities who experience disparities in healthcare access and health outcomes. She channeled her diverse experience and passion for innovation into founding and leading a research and development program at a global biotechnology company that is now fully dedicated to ensuring that clinical research is not only more inclusive but also more impactful for patients, especially those who have historically been excluded from participating in research.
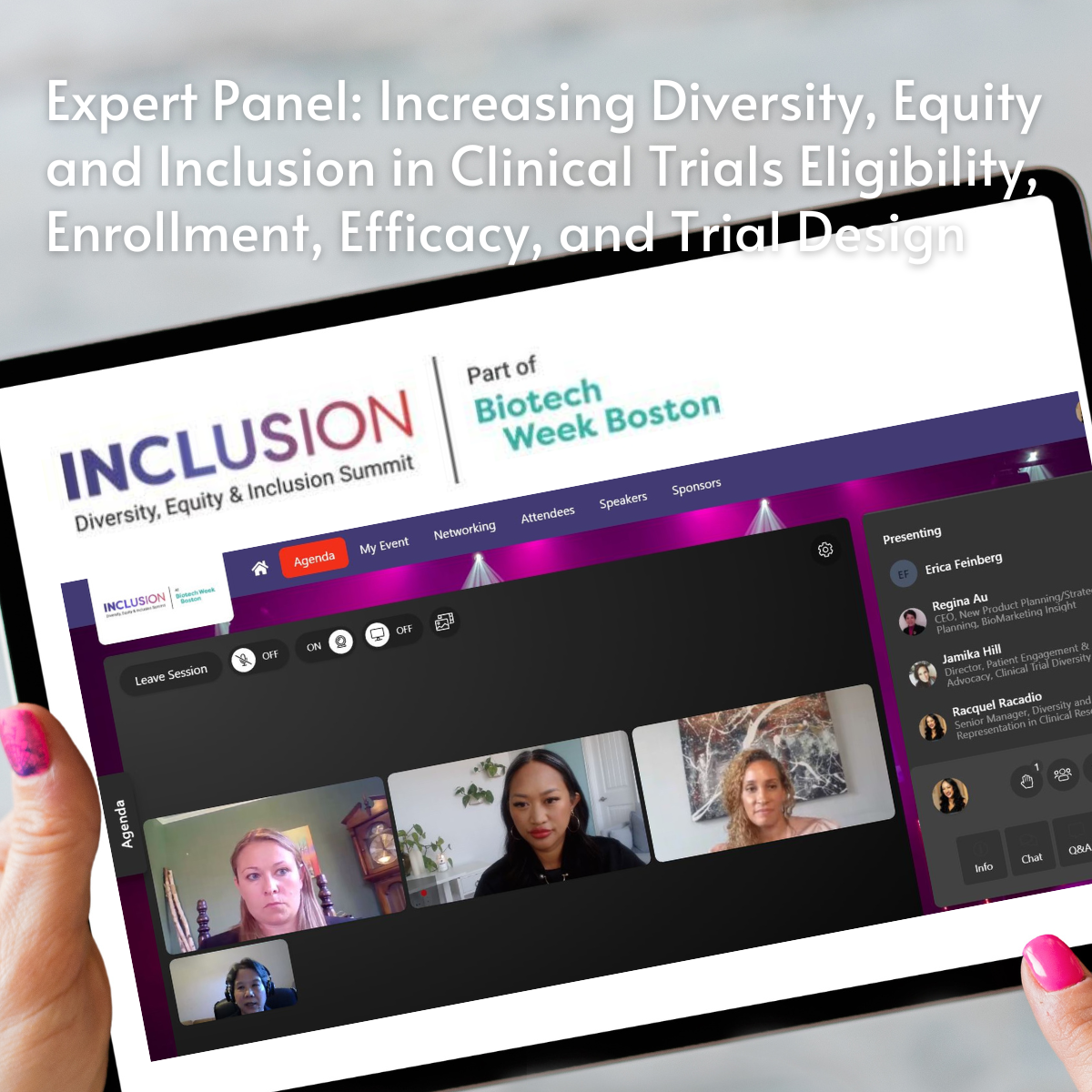
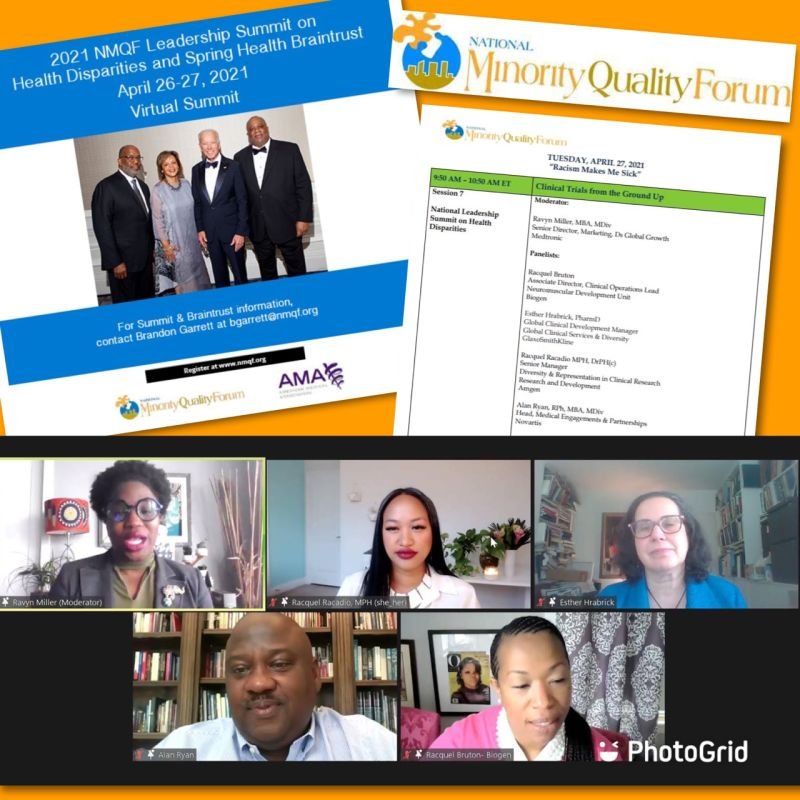
Racquel is a subject matter expert and thought leader on the topics of clinical trial diversity and representation, health equity, and diversity, equity, inclusion, and belonging (DEIB), serving as a speaker and panelist for national and global events, an author, contributor, and reviewer for peer-reviewed journals, and an advisor to cross-industry consortia and collaborations.
Racquel is a graduate of the Doctor of Public Health (DrPH) program at the University of North Caroling at Chapel Hill Gillings School of Public Health, where her dissertation research studied how technology may reduce barriers to oncology clinical trial participation for participants from racialized and minoritized communities. With strong roots in Wisconsin, she also holds a Master of Public Health (MPH) degree from the Medical College of Wisconsin and bachelor’s degrees in Biological Sciences (BS) from the University of Wisconsin-Milwaukee and in Journalism (JBA) from the University of Wisconsin-Madison.
-

Management Consulting
Program Management. Project Management. Change Management. Process Optimization.
-

Clinical Research
Clinical Development Operations. Clinical Study Design and Feasibility. Clinical Trial Oversight. Site Start-Up and Monitoring. Site Management and Investigator Engagement. Diversity, Inclusion, and Representation of Participants, Caregivers, Investigators, and Site Staff.
-

Communications
Copywriting and Editing. Presentation Development. Corporate Communications. Brand and Image Strategy. Internal and External.
Let's talk about the important things
Let's talk about the important things
SPEAKING
Racquel is a subject matter expert and thought leader on the topics of clinical trial diversity and representation, health equity, and diversity, equity, inclusion, and belonging (DEIB), serving as a speaker and panelist for national and global events, an author, contributor, and reviewer for peer-reviewed journals, and an advisor to cross-industry consortia and collaborations.
-
Challenges to and approaches to improve diversity and representation of participants and investigators and site staff participating in clinical trials. Current topics related to human data, regulatory and policy landscape, industry trends, cross-industry collaboration, disparities in clinical research access, decentralized clinical trials, patient and community engagement.
-
Health equity topics including health care access and health outcomes, social determinants of health, and disparities related to race, ethnicity, sex, age, and other factors.
-
DEIB topics including racial, ethnic, sex, and gender-related issues in the workplace, in research, and society at large.
KEY TOPICS
Interested in Inviting Racquel to Speak or Participate on a Panel?
LISTEN. WATCH. READ.
LISTEN. WATCH. READ.
Select Media
Journal of Racial and Ethnic Health Disparities
Diversity and Representation Among United States Participants in Amgen Clinical Trials
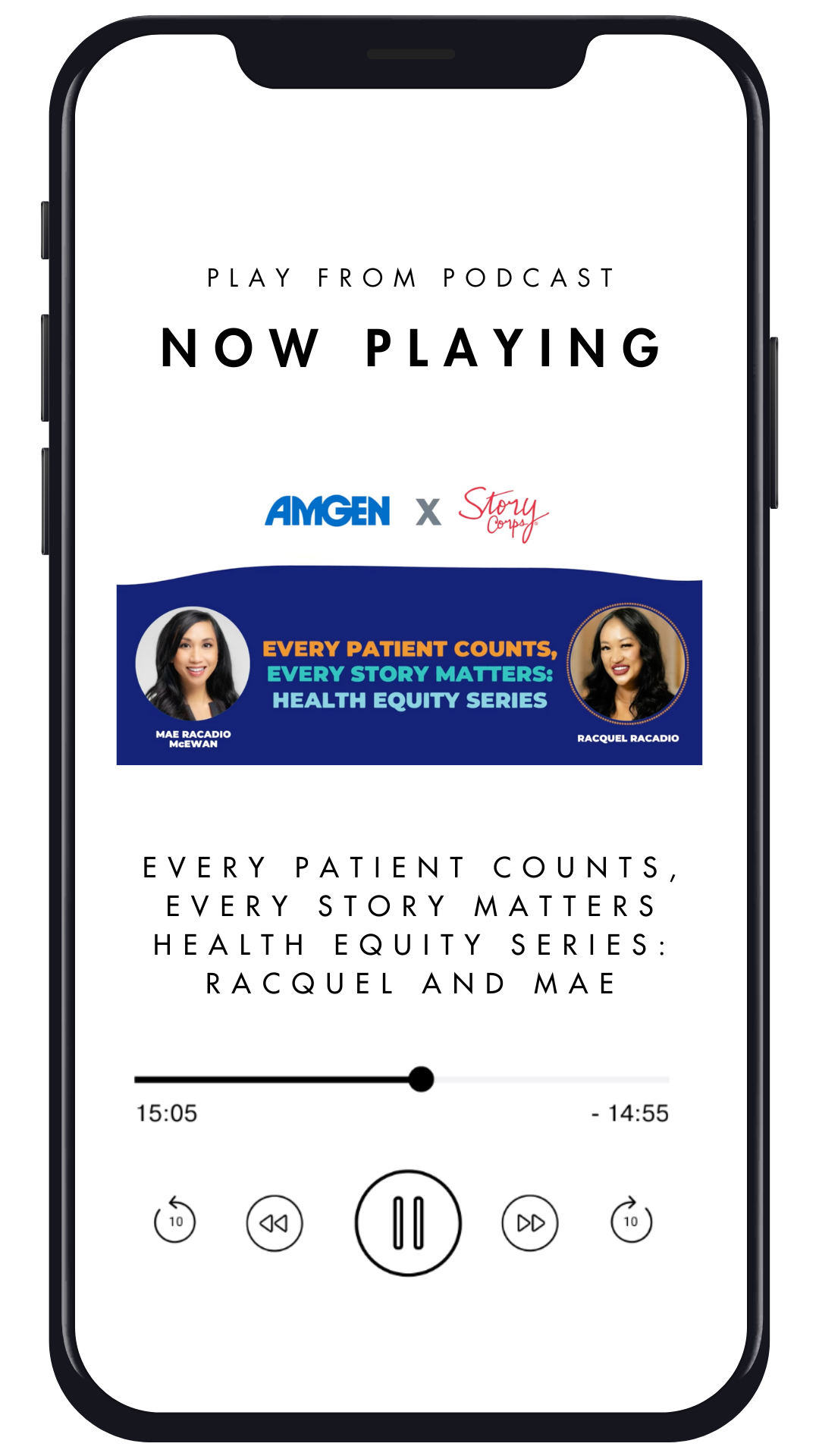
StoryCorps Podcast
Every patient counts, every story matters
The Atlantic Health Equity Summit
What does equitable healthcare look like?
Digital Medicine Society
How can we be accountable for diversity and inclusion in digitized clinical trials?
USC Marshall Center for Effective Organizations
The potential for ERGs to drive business and community impact